These modules include short lecture videos, animations, and relevant OpenStax Chemistry readings for a general chemistry for science majors course.
- Subject:
- Biology
- Chemistry
- Physical Science
- Material Type:
- Textbook
- Date Added:
- 10/19/2020

These modules include short lecture videos, animations, and relevant OpenStax Chemistry readings for a general chemistry for science majors course.
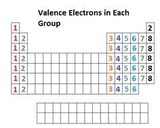
This multi-part module introduces covalent bonding and Lewis structures as a model of covalent bonding.Starting with valence electrons, a method of connecting unpaired electrons and/or redistributing valence electrons to satisfy the octet rule is introduced.Numerous examples are presented including CO, ozone, and polyatomic ions
This module is an introduction to using VSEPR to predict molecular shape based on Lewis structures. It is limited to steric numbers of 6 or less.
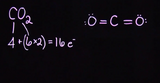
Step-by-step creation of two example Lewis structures: water and carbon dioxide. 1st step: Count and sum valence electrons. 2nd step: Determine central atom and connect to other atoms. 3rd step: Fill valence shells of outer atoms. 4th step: Fill valence shell on central atom.

"This course provides an introduction to the chemistry of biological, inorganic, and organic molecules.ĺĘTheĺĘemphasis isĺĘon basic principles of atomic and molecular electronic structure, thermodynamics, acid-base and redox equilibria, chemical kinetics, and catalysis. In an effort to illuminate connections between chemistry and biology, a list of the biology-, medicine-, and MIT research-related examples used in 5.111 is provided in Biology-Related Examples. Acknowledgements Development and implementation of the biology-related materials in this course were funded through an HHMI Professors grant to Prof. Catherine L. Drennan."