292 Results


This module introduces balancing chemical reactions with stoichiometric coefficients. Includes a link to an interactive website for practicing balancing reactions, a video showing specific practice problems, and a video with animations as visual aids demonstrating why we balance chemical reactions.
This module is an introduction to the relationship between stoichiometric coefficients, limiting and excess reactant, and theoretical yield. Includes a worked example asking for theoretical yield given two starting masses, to identify the limiting reactant, and calculate the percent yield from a given hypothetical "actual" yield.

This module has two parts: an introduction (rules) to predicting products based on the 5 reaction types; and a video with three specific examples.This module does not cover net ionic equations, balancing, or phase designations.

This module includes solubility rules, how to use the solubility rules, calculating moles of ions from grams of compound, and how to write Net Ionic Equations.

This module gives an overview of the main types of chemical reactions in first-semester general chemistry:1- Combination2- Decomposition3- Single Displacement4- Double Displacement5- Combustion
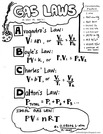
This module covers gases from properties and Kinetic Molecular Theory to the ABC gas laws, the Ideal Gas Law, and Dalton's Law of Partial Pressures.
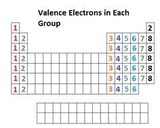
This multi-part module introduces covalent bonding and Lewis structures as a model of covalent bonding.Starting with valence electrons, a method of connecting unpaired electrons and/or redistributing valence electrons to satisfy the octet rule is introduced.Numerous examples are presented including CO, ozone, and polyatomic ions
This module is an introduction to using VSEPR to predict molecular shape based on Lewis structures. It is limited to steric numbers of 6 or less.
A three-quarter general chemistry sequence primarily for science, pre-professional, and engineering students. The CHEM& 161/162/163 series introduces the basic concepts of chemistry: atomic structure and bonding, periodicity, physical measurement, quantitative relationships, chemical reactivity, oxidation and reduction, stoichiometry, ideal gas laws, aqueous solutions, colligative properties, intermolecular forces, structure of matter, equilibrium, acid/base topics, kinetics, thermodynamics, electrochemistry, nuclear chemistry, qualitative analysis, d-block metals and coordination chemistry, and an introduction to organic chemistry.Login: guest_oclPassword: ocl
- Subject:
- Chemistry
- Physical Science
- Material Type:
- Activity/Lab
- Full Course
- Homework/Assignment
- Lecture Notes
- Lesson Plan
- Reading
- Syllabus
- Provider:
- Washington State Board for Community & Technical Colleges
- Provider Set:
- Open Course Library
- Date Added:
- 10/31/2011

"Global Climate Change through Geologic Time" is a remix with enhancements that fits within content I implement with my students called the "Global Change Modern and Paleo Considerations" module, for example. My adopted then adapted expanded item is an extension of earlier modular activities I designed to do with students including How Science Works from Howard Hughes Medical Institute (HHMI) and using the HHMI Earth Explorer interactive to consider data trends through time that my classes do earlier in a semester. There is also a series of embedded, supporting ADA Compliant streaming videos used and I also include engagement through the UCMP Understanding Science interactive platform.
- Subject:
- Atmospheric Science
- Biology
- Chemistry
- Ecology
- Environmental Science
- Geology
- Oceanography
- Open Educational Resources & Practice
- Material Type:
- Diagram/Illustration
- Interactive
- Lecture Notes
- Lesson
- Reading
- Author:
- Wendi J. W. Williams, PhD
- Date Added:
- 07/19/2023
Fundamental principles of biochemistry. Analysis of the mode of action and structure of regulatory, binding, and catalytic proteins. The tools and analytical methods that biochemists use to dissect biological problems. Analysis of the mode of action and structure of regulatory, binding, and catalytic proteins.
- Subject:
- Biology
- Chemistry
- Life Science
- Physical Science
- Material Type:
- Full Course
- Provider:
- MIT
- Provider Set:
- MIT OpenCourseWare
- Author:
- Frank Solomon
- Solomon, Frank
- Date Added:
- 01/01/2001
This course uses reaction kinetics, batch reactor analysis, batch distillation, batch operations scheduling, safety analysis, and the ABACUSS process simulator to introduce process design and analysis techniques.
- Subject:
- Chemistry
- Physical Science
- Material Type:
- Full Course
- Provider:
- MIT
- Provider Set:
- MIT OpenCourseWare
- Author:
- Johnston, Barry
- Date Added:
- 01/01/2006
Presents and solves chemical engineering problems in an industrial context, with applications varying by semester. Emphasis on the integration of fundamental concepts with approaches of process design. Emphasis on problems that demand synthesis, economic analysis, and process design .This course introduces students to methods and background needed for the conceptual design of continuously operating chemical plants. Particular attention is paid to the use of process modeling tools such as Aspen that are used in industry and to problems of current interest. Each student team is assigned to evaluate and design a different technology and prepare a final design report. For spring 2006, the theme of the course is to design technologies for lowering the emissions of climatically active gases from processes that use coal as the primary fuel.
- Subject:
- Chemistry
- Engineering
- Physical Science
- Material Type:
- Full Course
- Provider:
- MIT
- Provider Set:
- MIT OpenCourseWare
- Author:
- Mcrae, Gregory
- Date Added:
- 01/01/2006

This course provides a brief introduction to the field of biocatalysis in the context of process design. Fundamental topics include why and when one may choose to use biological systems for chemical conversion, considerations for using free enzymes versus whole cells, and issues related to design and development of bioconversion processes. Biological and engineering problems are discussed as well as how one may arrive at both biological and engineering solutions.
- Subject:
- Chemistry
- Engineering
- Physical Science
- Material Type:
- Full Course
- Provider:
- MIT
- Provider Set:
- MIT OpenCourseWare
- Author:
- Prather, Kristala
- Date Added:
- 01/01/2004

This resource contains a video covering integrated rate laws. Zero, first and second order equations are derived. Example problems showing graphical method of determination are shown. Sample calculations using first and second order equations are shown.This resource corresponds to Chemistry Atoms First 2e section 17.4https://openstax.org/books/chemistry-atoms-first-2e/pages/17-4-integrated-rate-laws
- Subject:
- Chemistry
- Material Type:
- Lecture
- Lecture Notes
- Author:
- Jane Johnson-Carr
- Date Added:
- 05/27/2021

This module includes an interactive acid-base titration that allows students to select an acid (general "strong" or several weak), initial concentration of the acid and base, and initial volume of the acid. Then, students click on the virtual stopcock to add base.The curve is generated while the correct equation is highlighted for each part of the titration.
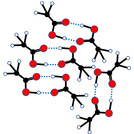
Melting Point2. Animation of breaking of intermolecular forces in acetic acid molecule at three different temperatures: low, moderate, highAcetic acid molecules move slowest at low temperature; occupy least volume; have all blue intermolecular forces Acetic acid molecules move faster at moderate temperature; occupy more volume; have some blue intermolecular forces brokenAcetic acid molecules move fastest at high temperature; occupy large volume; have no blue intermolecular forces-water molecules move faster since lighter-acetic acid molecules move slower since heavier
- Subject:
- Biochemistry
- Chemistry
- Material Type:
- Interactive
- Author:
- Nicol Alexander
- Date Added:
- 12/15/2021
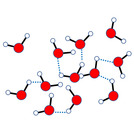
Melting point1. Animation of breaking of intermolecular forces in water molecule at three different temperatures: low, moderate, highWater molecules move slowest at low temperature; occupy least volume; have all blue intermolecular forces Water molecules move faster at moderate temperature; occupy more volume; have some blue intermolecular forces brokenWater molecules move fastest at high temperature; occupy large volume; have no blue intermolecular forces
- Subject:
- Biochemistry
- Chemistry
- Material Type:
- Interactive
- Author:
- Nicol Alexander
- Date Added:
- 12/15/2021