
Short succinct explanation of the basics of chemistry in easy to use language and examples
- Subject:
- Chemistry
- Physical Science
- Material Type:
- Textbook
- Provider:
- eCampusOntario
- Author:
- Michael Mombourquette
- Date Added:
- 08/09/2021

Short succinct explanation of the basics of chemistry in easy to use language and examples
Properties of gases can be modeled using some relatively simple equations, which we can relate to the behavior of individual gas molecules. We will learn about the ideal gas law, vapor pressure, partial pressure, and the Maxwell Boltzmann distribution.
An integrated course stressing the principles of biology. Life processes are examined primarily at the molecular and cellular levels. Intended for students majoring in biology or for non-majors who wish to take advanced biology courses.
This is a General Chemistry 1 course taught at Sowela Technical Community College. This course utilizes Openstax Chemistry resources with added videos, powerpoint slides, and assessments.
Chemistry is designed to meet the scope and sequence requirements of the two-semester general chemistry course. The textbook provides an important opportunity for students to learn the core concepts of chemistry and understand how those concepts apply to their lives and the world around them. The book also includes a number of innovative features, including interactive exercises and real-world applications, designed to enhance student learning.
These are OER labs created for General Chemistry II courses.
This material is based on key universal learning objectives for General Chemistry II (CHEM 1412) course. They are adaptable for various textbooks and include images sourced from Wikimedia Commons, Openstax.org, or those created by myself. DRAFTS.
This course provides an opportunity for students to learn the core concepts of chemistry and understand how those concepts apply to their lives and the world around them, meeting the scope and sequence of most general chemistry courses.

The overall goal of the authors with General Chemistry: Principles, Patterns, and Applications was to produce a text that introduces the students to the relevance and excitement of chemistry.Although much of first-year chemistry is taught as a service course, Bruce and Patricia feel there is no reason that the intrinsic excitement and potential of chemistry cannot be the focal point of the text and the course. So, they emphasize the positive aspects of chemistry and its relationship to studentsŐ lives, which requires bringing in applications early and often. In addition, the authors feel that many first year chemistry students have an enthusiasm for biologically and medically relevant topics, so they use an integrated approach in their text that includes explicit discussions of biological and environmental applications of chemistry.
The overall goal of the authors with General Chemistry: Principles, Patterns, and Applications was to produce a text that introduces the students to the relevance and excitement of chemistry.
Although much of first-year chemistry is taught as a service course, Bruce and Patricia feel there is no reason that the intrinsic excitement and potential of chemistry cannot be the focal point of the text and the course. So, they emphasize the positive aspects of chemistry and its relationship to students' lives, which requires bringing in applications early and often. In addition, the authors feel that many first year chemistry students have an enthusiasm for biologically and medically relevant topics, so they use an integrated approach in their text that includes explicit discussions of biological and environmental applications of chemistry.
Topics relevant to materials science are also introduced to meet the more specific needs of engineering students. To facilitate integration of such material, simple organic structures, nomenclature, and reactions are introduced very early in the text, and both organic and inorganic examples are used wherever possible. This approach emphasizes the distinctions between ionic and covalent bonding, thus enhancing the students' chance of success in the organic chemistry course that traditionally follows general chemistry. Finally, the authors made a conscious effort to treat material that has traditionally been relegated to boxes, and thus perhaps perceived as peripheral by the students, by incorporating it into the text to serve as a learning tool.
These modules include short lecture videos, animations, and relevant OpenStax Chemistry readings for a general chemistry for science majors course.

This Module teaches how to convert mass % to empirical formula, how to identify empirical and molecular formulas, and how to calculate molar mass of a compound.

This module is a brief introduction to the definitions of mixture, compound, and element and includes a Ted Ed video on What's in a Mixture: the science of macaroni salad.
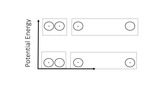
This module is a brief introduction to making a distinction between kinetic and potential energy. The laws of potential energy inform many of our definitions later in chemistry and are especially useful when related specifically to charges.

This module has two parts:1- Introduction to the concept of a "mole" and Avogadro's number.2- Application of the Mole (Avogadro's number) as a conversion factor relating mass in grams to number of atoms or molecules (formula unit for ionic compounds)

This module teaches the basic rules of naming binary compounds for general chemistry. Hydrocarbons and acids are not included.
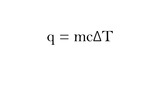
This module includes a mini-lecture (5 minutes) that defines specific heat capacity and includes an examples (no unit conversions).

This is a fun way to engage students on paradoxes or misconceptions about physical properties. This module specifically targets common misconceptions about temperature, heat, and thermal conductivity.

This module is an introduction to Accuracy, Precision, and Sig figs. It includes how to count the number of significant digits and defining accuracy versus precision. Outcomes:1- Students will identify the number of significant digits2- Students will define accuracy and precision