
This module teaches the basic rules of naming binary compounds for general chemistry. Hydrocarbons and acids are not included.

This module teaches the basic rules of naming binary compounds for general chemistry. Hydrocarbons and acids are not included.
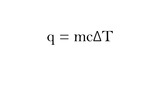
This module includes a mini-lecture (5 minutes) that defines specific heat capacity and includes an examples (no unit conversions).

This is a fun way to engage students on paradoxes or misconceptions about physical properties. This module specifically targets common misconceptions about temperature, heat, and thermal conductivity.

This module is an introduction to Accuracy, Precision, and Sig figs. It includes how to count the number of significant digits and defining accuracy versus precision. Outcomes:1- Students will identify the number of significant digits2- Students will define accuracy and precision

This module introduces balancing chemical reactions with stoichiometric coefficients. Includes a link to an interactive website for practicing balancing reactions, a video showing specific practice problems, and a video with animations as visual aids demonstrating why we balance chemical reactions.
This module is an introduction to the relationship between stoichiometric coefficients, limiting and excess reactant, and theoretical yield. Includes a worked example asking for theoretical yield given two starting masses, to identify the limiting reactant, and calculate the percent yield from a given hypothetical "actual" yield.

This module has two parts: an introduction (rules) to predicting products based on the 5 reaction types; and a video with three specific examples.This module does not cover net ionic equations, balancing, or phase designations.

This module includes solubility rules, how to use the solubility rules, calculating moles of ions from grams of compound, and how to write Net Ionic Equations.

This module gives an overview of the main types of chemical reactions in first-semester general chemistry:1- Combination2- Decomposition3- Single Displacement4- Double Displacement5- Combustion
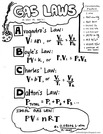
This module covers gases from properties and Kinetic Molecular Theory to the ABC gas laws, the Ideal Gas Law, and Dalton's Law of Partial Pressures.
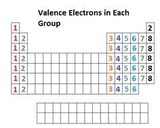
This multi-part module introduces covalent bonding and Lewis structures as a model of covalent bonding.Starting with valence electrons, a method of connecting unpaired electrons and/or redistributing valence electrons to satisfy the octet rule is introduced.Numerous examples are presented including CO, ozone, and polyatomic ions
This module is an introduction to using VSEPR to predict molecular shape based on Lewis structures. It is limited to steric numbers of 6 or less.
A three-quarter general chemistry sequence primarily for science, pre-professional, and engineering students. The CHEM& 161/162/163 series introduces the basic concepts of chemistry: atomic structure and bonding, periodicity, physical measurement, quantitative relationships, chemical reactivity, oxidation and reduction, stoichiometry, ideal gas laws, aqueous solutions, colligative properties, intermolecular forces, structure of matter, equilibrium, acid/base topics, kinetics, thermodynamics, electrochemistry, nuclear chemistry, qualitative analysis, d-block metals and coordination chemistry, and an introduction to organic chemistry.Login: guest_oclPassword: ocl
Generate electricity with a bar magnet! Discover the physics behind the phenomena by exploring magnets and how you can use them to make a bulb light.
How do greenhouse gases affect the climate? Explore the atmosphere during the ice age and today. What happens when you add clouds? Change the greenhouse gas concentration and see how the temperature changes. Then compare to the effect of glass panes. Zoom in and see how light interacts with molecules. Do all atmospheric gases contribute to the greenhouse effect?

This module includes an interactive acid-base titration that allows students to select an acid (general "strong" or several weak), initial concentration of the acid and base, and initial volume of the acid. Then, students click on the virtual stopcock to add base.The curve is generated while the correct equation is highlighted for each part of the titration.
" The fundamental concepts, and approaches of aerospace engineering, are highlighted through lectures on aeronautics, astronautics, and design. Active learning aerospace modules make use of information technology. Student teams are immersed in a hands-on, lighter-than-air (LTA) vehicle design project, where they design, build, and fly radio-controlled LTA vehicles. The connections between theory and practice are realized in the design exercises. Required design reviews precede the LTA race competition. The performance, weight, and principal characteristics of the LTA vehicles are estimated and illustrated using physics, mathematics, and chemistry known to freshmen, the emphasis being on the application of this knowledge to aerospace engineering and design rather than on exposure to new science and mathematics."

" This class is a project-based introduction to the engineering of synthetic biological systems. Throughout the term, students develop projects that are responsive to real-world problems of their choosing, and whose solutions depend on biological technologies. Lectures, discussions, and studio exercises will introduce (1) components and control of prokaryotic and eukaryotic behavior, (2) DNA synthesis, standards, and abstraction in biological engineering, and (3) issues of human practice, including biological safety; security; ownership, sharing, and innovation; and ethics. Enrollment preference is given to freshmen. This subject was originally developed and first taught in Spring 2008 by Drew Endy and Natalie Kuldell. Many of Drew's materials are used in this Spring 2009 version, and are included with his permission. This OCW Web site is based on the OpenWetWare class Wiki, found at OpenWetWare: 20.020 (S09)"