This module is a brief introduction to making a distinction between kinetic and potential energy. The laws of potential energy inform many of our definitions later in chemistry and are especially useful when related specifically to charges.

Resources for the Earth, Life, and Natural Sciences Division

This module is a brief introduction to making a distinction between kinetic and potential energy. The laws of potential energy inform many of our definitions later in chemistry and are especially useful when related specifically to charges.

This module has two parts:1- Introduction to the concept of a "mole" and Avogadro's number.2- Application of the Mole (Avogadro's number) as a conversion factor relating mass in grams to number of atoms or molecules (formula unit for ionic compounds)

This module teaches the basic rules of naming binary compounds for general chemistry. Hydrocarbons and acids are not included.
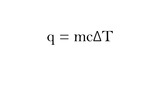
This module includes a mini-lecture (5 minutes) that defines specific heat capacity and includes an examples (no unit conversions).

This is a fun way to engage students on paradoxes or misconceptions about physical properties. This module specifically targets common misconceptions about temperature, heat, and thermal conductivity.

This module is an introduction to Accuracy, Precision, and Sig figs. It includes how to count the number of significant digits and defining accuracy versus precision. Outcomes:1- Students will identify the number of significant digits2- Students will define accuracy and precision

This module introduces balancing chemical reactions with stoichiometric coefficients. Includes a link to an interactive website for practicing balancing reactions, a video showing specific practice problems, and a video with animations as visual aids demonstrating why we balance chemical reactions.
This module is an introduction to the relationship between stoichiometric coefficients, limiting and excess reactant, and theoretical yield. Includes a worked example asking for theoretical yield given two starting masses, to identify the limiting reactant, and calculate the percent yield from a given hypothetical "actual" yield.

This module has two parts: an introduction (rules) to predicting products based on the 5 reaction types; and a video with three specific examples.This module does not cover net ionic equations, balancing, or phase designations.

This module includes solubility rules, how to use the solubility rules, calculating moles of ions from grams of compound, and how to write Net Ionic Equations.

This module gives an overview of the main types of chemical reactions in first-semester general chemistry:1- Combination2- Decomposition3- Single Displacement4- Double Displacement5- Combustion
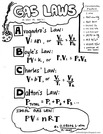
This module covers gases from properties and Kinetic Molecular Theory to the ABC gas laws, the Ideal Gas Law, and Dalton's Law of Partial Pressures.
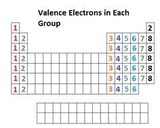
This multi-part module introduces covalent bonding and Lewis structures as a model of covalent bonding.Starting with valence electrons, a method of connecting unpaired electrons and/or redistributing valence electrons to satisfy the octet rule is introduced.Numerous examples are presented including CO, ozone, and polyatomic ions
This module is an introduction to using VSEPR to predict molecular shape based on Lewis structures. It is limited to steric numbers of 6 or less.
A three-quarter general chemistry sequence primarily for science, pre-professional, and engineering students. The CHEM& 161/162/163 series introduces the basic concepts of chemistry: atomic structure and bonding, periodicity, physical measurement, quantitative relationships, chemical reactivity, oxidation and reduction, stoichiometry, ideal gas laws, aqueous solutions, colligative properties, intermolecular forces, structure of matter, equilibrium, acid/base topics, kinetics, thermodynamics, electrochemistry, nuclear chemistry, qualitative analysis, d-block metals and coordination chemistry, and an introduction to organic chemistry.Login: guest_oclPassword: ocl
Four types of OER material are found here: Study Guides, Laboratory Documents, XML Moodle Quiz Question Files, and YouTube screencast videos. These materials have been used in both calculus and non- calculus Introductory Physics. Twelve to fourteen different topics are covered over a fourteen week semester. Most topics are supported by a Study Guide, a laboratory, a set of Moodle Quiz formatted questions, and one or more YouTube posted screencast videos. These posted resources are at various levels of completeness. Some remain in rough draft stage. OpenStax Physics textbooks are used in all courses.

This is a textbook on general relativity for upper-division undergraduates majoring in physics, at roughly the same level as Rindler's Essential Relativity or Hartle's Gravity. The book is meant to be especially well adapted for self-study, and answers are given in the back of the book for almost all the problems. The ratio of conceptual to mathematical problems is higher than in most books. The focus is on "index-gymnastics" techniques, to the exclusion of index-free notation. Knowledge of first-year calculus and lower-division mechanics and electromagnetism is assumed. Special relativity is introduced from scratch, but it will be very helpful to have a thorough previous knowledge of SR, at the level of a book such as Taylor and Wheeler's Spacetime Physics or my own text Special Relativity.
Generate electricity with a bar magnet! Discover the physics behind the phenomena by exploring magnets and how you can use them to make a bulb light.