This resource includes readings from OpenStax Chemistry 2e, two mini-lectures, and a Ted Ed video on the conceptual understanding of the relative strengths of acids and bases.

This resource includes readings from OpenStax Chemistry 2e, two mini-lectures, and a Ted Ed video on the conceptual understanding of the relative strengths of acids and bases.
" The fundamental concepts, and approaches of aerospace engineering, are highlighted through lectures on aeronautics, astronautics, and design. Active learning aerospace modules make use of information technology. Student teams are immersed in a hands-on, lighter-than-air (LTA) vehicle design project, where they design, build, and fly radio-controlled LTA vehicles. The connections between theory and practice are realized in the design exercises. Required design reviews precede the LTA race competition. The performance, weight, and principal characteristics of the LTA vehicles are estimated and illustrated using physics, mathematics, and chemistry known to freshmen, the emphasis being on the application of this knowledge to aerospace engineering and design rather than on exposure to new science and mathematics."

" This class is a project-based introduction to the engineering of synthetic biological systems. Throughout the term, students develop projects that are responsive to real-world problems of their choosing, and whose solutions depend on biological technologies. Lectures, discussions, and studio exercises will introduce (1) components and control of prokaryotic and eukaryotic behavior, (2) DNA synthesis, standards, and abstraction in biological engineering, and (3) issues of human practice, including biological safety; security; ownership, sharing, and innovation; and ethics. Enrollment preference is given to freshmen. This subject was originally developed and first taught in Spring 2008 by Drew Endy and Natalie Kuldell. Many of Drew's materials are used in this Spring 2009 version, and are included with his permission. This OCW Web site is based on the OpenWetWare class Wiki, found at OpenWetWare: 20.020 (S09)"
From consumer products to space-age technologies, chemistry affects our daily lives. In this course, students will learn the structure of matter and how it behaves under various conditions in order to better understand the chemical world. Designed for students with little or no chemistry background. Laboratory activities extend lecture concepts and introduce students to the experimental process. This course is designed for a face-to-face mode of instruction using online resources. Course content is divided into units. Each unit may include text readings, laboratory preparation, study questions, thought-provoking discussions, written assignments, learning activities, and group projects.Login: guest_oclPassword: ocl

This course is the first part of a modular sequence of increasingly sophisticated (and challenging) laboratory courses required of all Chemistry majors: 5.35 Introduction to Experimental Chemistry, 5.36 Biochemistry and Organic Laboratory, 5.37 Organic and Inorganic Laboratory, and 5.38 Physical Chemistry Laboratory. This course provides students with a survey of spectroscopy, and introduces synthesis of coordination compounds and kinetics. This class is part of the new laboratory curriculum in the MIT Department of Chemistry. Undergraduate Research-Inspired Experimental Chemistry Alternatives (URIECA) introduces students to cutting edge research topics in a modular format. AcknowledgementsProfessor Nelson and Dr. Twardowski would like to acknowledge the contributions of MIT Professor Timothy Swager to the development of this course.
" This course, which spans a third of a semester, provides students with experienceĺĘusing techniques employed in synthetic organic chemistry. It alsoĺĘintroduces them to the exciting research area of catalytic chiral catalysis. This class is part of the new laboratory curriculum in the MIT Department of Chemistry. Undergraduate Research-Inspired Experimental Chemistry Alternatives (URIECA) introduces students to cutting edge research topics in a modular format."
This resource contains two mini-lecture videos that tie into OpenStax Chemistry Atoms-First 2e chapter 11.

This resource has the following Outcomes:Students will relate physical and chemical properties to groups and periodsStudents will determine the number of protons from an atomic numberStudents will determine the number of neutrons from an isotope mass number and atomic number
David W. Ball of Cleveland State University brings his new survey of general chemistry text, Introductory Chemistry, to the market with a fresh theme that will be sure to hold student interest: "Chemistry is Everywhere." Introductory Chemistry is intended for a one-semester introductory or preparatory chemistry course. Throughout the chapters, David presents two features that reinforce the theme of the textbook, that chemistry is everywhere.
The first is the boxed feature titled, appropriately, ”Chemistry is Everywhere“. This feature takes a topic of the chapter and demonstrates how this topic shows up in everyday life. In the introductory chapter, ”Chemistry is Everywhere“ focuses on the personal hygiene products that students may use every morning: toothpaste, soap, shampoo among others. These products are chemicals, aren't they? This book explores some of the chemical reactions like the ones that give students clean and healthy teeth, and shiny hair. This feature makes it clear to students that chemistry is, indeed, everywhere, and it will promote student retention in what is sometimes considered an intimidating course.
The second boxed feature focuses on chemistry that students likely indulge in every day: eating and drinking. In the ”Food and Drink App“, David discusses how the chemistry of the chapter applies to things that students eat and drink every day. Carbonated beverages depend on the behavior of gases, foods contain acids and bases, and everyone actually eats certain rocks. (Yikes!) Cooking, eating, drinking, metabolism — all chemical processes students are involved with all the time.
These features allow students to see the things we interact with every day in a new light — as chemistry.
Just like many of the one-semester chemistry books you may be used to, each section in David Ball's starts with one or more Learning Objectives, which list the main points of the section. Each section ends with Key Takeaways, which are reviews of the main points of the section. Each chapter is full of examples to illustrate the key points of the materials, and each example is followed with a similar ”Test Yourself“ exercise to see if the student understands the concept. Each section ends with its own set of paired exercises to practice the material from that section, and each chapter ends with a section of ”Additional Exercises“ that are more challenging or require multiple steps or skills to answer.

David W. Ball of Cleveland State University brings his new survey of general chemistry text, Introductory Chemistry, to the market with a fresh theme that will be sure to hold student interest: "Chemistry is Everywhere." Introductory Chemistry is intended for a one-semester introductory or preparatory chemistry course. Throughout the chapters, David presents two features that reinforce the theme of the textbook, that chemistry is everywhere.The first is the boxed feature titled, appropriately, “Chemistry is Everywhere”. This feature takes a topic of the chapter and demonstrates how this topic shows up in everyday life. In the introductory chapter, “Chemistry is Everywhere” focuses on the personal hygiene products that students may use every morning: toothpaste, soap, shampoo among others. These products are chemicals, aren’t they? This book explores some of the chemical reactions like the ones that give students clean and healthy teeth, and shiny hair. This feature makes it clear to students that chemistry is, indeed, everywhere, and it will promote student retention in what is sometimes considered an intimidating course.The second boxed feature focuses on chemistry that students likely indulge in every day: eating and drinking. In the “Food and Drink App”, David discusses how the chemistry of the chapter applies to things that students eat and drink every day. Carbonated beverages depend on the behavior of gases, foods contain acids and bases, and everyone actually eats certain rocks. (Yikes!) Cooking, eating, drinking, metabolism – all chemical processes students are involved with all the time. These features allow students to see the things we interact with every day in a new light – as chemistry.Just like many of the one-semester chemistry books you may be used to, each section in David Ball's <="" em=""> starts with one or more Learning Objectives, which list the main points of the section. Each section ends with Key Takeaways, which are reviews of the main points of the section. Each chapter is full of examples to illustrate the key points of the materials, and each example is followed with a similar “Test Yourself” exercise to see if the student understands the concept. Each section ends with its own set of paired exercises to practice the material from that section, and each chapter ends with a section of “Additional Exercises” that are more challenging or require multiple steps or skills to answer.David took the time to treat mathematical problems in Introductory Chemistry one of two ways, either as a conversion-factor problem or as a formula problem. David believes having two basic mathematical approaches (converting and formulas) allows the text to focus on the logic of the approach and not tricks or shortcuts; which speaks to the final point about Introductory Chemistry.You'll notice that David took no shortcuts with the material in this text, his inviting writing style, concise approach, consistent presentation, and interesting pedagogy have given it some of the best peer reviews we've seen at Flat World. So, order a desk copy or dive in now to see for yourself.
This survey should give you enough knowledge to appreciate the impact of chemistry in everyday life and, if necessary, prepare you for additional instruction in chemistry. Throughout each chapter, I present two features that reinforce the theme of the textbookthat chemistry is all around you. The first is a feature titled, appropriately, Chemistry Is Everywhere. Chemistry Is Everywhere focuses on the personal hygiene products that you may use every morning: toothpaste, soap, and shampoo, among others. These products are chemicals, arent they? Ever wonder about the chemical reactions that they undergo to give you clean and healthy teeth or shiny hair? I will explore some of these chemical reactions in future chapters. But this feature makes it clear that chemistry is, indeed, everywhere. The other feature focuses on chemistry that you likely indulge in every day: eating and drinking. In the Food and Drink App, I discuss how the chemistry of the chapter applies to things that you eat and drink every day. Carbonated beverages depend on the behavior of gases, foods contain acids and bases, and we actually eat certain rocks. (Can you guess which rocks without looking ahead?) Cooking, eating, drinking, and metabolismwe are involved with all these chemical processes all the time. These two features allow us to see the things we interact with every day in a new lightas chemistry.
The goal of this textbook is not to make you an expert. True expertise in any field is a years-long endeavor. Here I will survey some of the basic topics of chemistry. This survey should give you enough knowledge to appreciate the impact of chemistry in everyday life and, if necessary, prepare you for additional instruction in chemistry.

The goal of this textbook is not to make you an expert. True expertise in any field is a years-long endeavor. Here I will survey some of the basic topics of chemistry. This survey should give you enough knowledge to appreciate the impact of chemistry in everyday life and, if necessary, prepare you for additional instruction in chemistry.
The goal of this textbook is not to make you an expert. True expertise in any field is a years-long endeavor. Here I will survey some of the basic topics of chemistry. This survey should give you enough knowledge to appreciate the impact of chemistry in everyday life and, if necessary, prepare you for additional instruction in chemistry.
The goal of this textbook is not to make you an expert. True expertise in any field is a years-long endeavor. Here I will survey some of the basic topics of chemistry. This survey should give you enough knowledge to appreciate the impact of chemistry in everyday life and, if necessary, prepare you for additional instruction in chemistry. Throughout each chapter, I present two features that reinforce the theme of the textbookthat chemistry is all around you. The first is a feature titled, appropriately, Chemistry Is Everywhere. Chemistry Is Everywhere focuses on the personal hygiene products that you may use every morning: toothpaste, soap, and shampoo, among others. These products are chemicals, arent they? Ever wonder about the chemical reactions that they undergo to give you clean and healthy teeth or shiny hair? I will explore some of these chemical reactions in future chapters. But this feature makes it clear that chemistry is, indeed, everywhere. The other feature focuses on chemistry that you likely indulge in every day: eating and drinking. In the Food and Drink App, I discuss how the chemistry of the chapter applies to things that you eat and drink every day. Carbonated beverages depend on the behavior of gases, foods contain acids and bases, and we actually eat certain rocks. (Can you guess which rocks without looking ahead?) Cooking, eating, drinking, and metabolismwe are involved with all these chemical processes all the time. These two features allow us to see the things we interact with every day in a new lightas chemistry.
The goal of this textbook is not to make you an expert. True expertise in any field is a years-long endeavor. Here I will survey some of the basic topics of chemistry. This survey should give you enough knowledge to appreciate the impact of chemistry in everyday life and, if necessary, prepare you for additional instruction in chemistry.
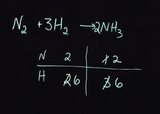
Example of balancing chemical reactions with ball and stick models showing CH4 and O2 reacting to form CO2 and H2O. Example on board of balancing N2 and H2 forming NH3 using a table.
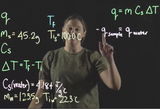
Discussion of heat, change in temperature, and specific heat capacity. Worked out example to determine the specific heat capacity of a sample via coffee cup calorimetry.

Changing units using conversion factors. One example of grams to pounds. Another example of miles per hour to kilometers per minute.

Naming simple compounds including binary type 1 and type 2 ionic compounds, binary covalent compounds, and acids.